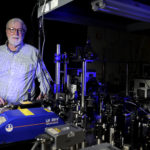
About Paul Champion
EDUCATION: PhD (Physics) University of Illinois; Postdoc (Physics/Chemistry) Cornell University
SCHOLARSHIP FOCUS: Experimental biological physics; Quantum biology; Time resolved Raman and femtosecond vibrational coherence spectroscopy; Ultrafast laser pump-probe methods; Wide dynamic range kinetics; Heme proteins; Proton and electron tunneling in biology.
HONORS AND AWARDS: Elected Fellow of the American Physical Society; Elected Fellow, American Association for the Advancement of Science; International Advisory Boards: Japan Ministry of Education, Culture, Sports, Science and Technology; Board of Directors Telluride Science Research Center; Fellow, Japanese Society for the Promotion of Science; NSF/CNRS Exchange Fellow; Advisory Board NSF Frontier Center; National Institutes of Health Career Development Award; NIH National Research Service Award; Standing Member of NIH Study Section on Molecular and Cellular Biophysics; Divisional Editor Physical Review Letters (Biological Physics); Executive Committee of American Physical Society Division of Biological Physics; Chair APS Biological Physics Award Committee; Lifetime Achievement Award: International Conference on Time Resolved Vibrational Spectroscopy.
RECENT RESEARCH PROJECTS:
“Electron-Nuclear Coupling, Charge Transport, and Catalysis in Biomolecules: The Role of Vibrational and Conformational Dynamics”, Principal Investigator, National Science Foundation.
“Femtosecond Stimulated Raman Scattering, Time Resolved Dynamics, and Electron‐Nuclear coupling in Biomolecules”, Principal Investigator, National Science Foundation.
“International Collaboration in Chemistry on Control of Excited State Proton Transfer in GFP”, Principal Investigator, National Science Foundation.
“Femtosecond Coherence Spectroscopy and Ultrafast Kinetic Investigations of Heme Proteins”, Principal Investigator, National Institutes of Health.
RECENT PUBLICATIONS:
“Electrical unfolding of cytochrome c during translocation through a nanopore constriction”, Prabhat Tripathi, Abdelkrim Benabbas, Behzad Mehrafrooz, Hirohito Yamazaki, Aleksei Aksimentiev, Paul M. Champion, and Meni Wanunu, Proc. Nat. Acad. Sci., 118 (17), e2016262118 (2021).
https://doi.org/10.1073/pnas.2016262118
“Proton Tunnelling and Proton-coupled Electron Transfer in Biological Systems: Theory and Experimental Analysis”, P. M. Champion and A. Benabbas, Theoretical and Computational Chemistry Series 18, Chap. 3, Tunnelling in Molecules: Nuclear Quantum Effects from Bio to Physical Chemistry, J. Kastner and S. Kozuch, Eds. Royal Soc. Chem. (2021).
https://doi.org/10.1039/9781839160370-00088
“All-Optical Helicity-Dependent Switching in Hybrid Metal–Ferromagnet Thin Films”. Feng Cheng, Zhidong Du, Xinjun Wang, Ziqiang Cai, Lin Li, Chuangtang Wang, Abdelkrim Benabbas, Paul Champion, Nianxiang Sun, Liang Pan, and Yongmin Liu, Adv. Optical Mater., 8, 2000379 (2020).
https://doi.org/10.1002/adom.202000379
“Adiabatic Ligand Binding in Heme Proteins: Ultrafast Kinetics of Methionine Rebinding in Ferrous Cytochrome c”, Abdelkrim Benabbas and Paul M. Champion, J. Phys. Chem. B, 122, 11431-11439 (2018).
http://dx.doi.org/10.1021/acs.jpcb.8b07355
“Proton-Coupled Electron Transfer and the Linear Approximation for Coupling to the Donor−Acceptor Distance Fluctuations”, Bridget Salna, Abdelkrim Benabbas, and Paul M. Champion, J. Phys. Chem. A, 121, 2199−2207 (2017). http://dx.doi.org/10.1021/acs.jpca.7b00539
The Champion lab studies the structure and dynamics of biomolecules using a variety of ultrafast laser-based techniques.