Northeastern University Distinguished Professor and Department of Biology Chair Jonathan Tilly has been successful in years of groundbreaking research in women’s health and fertility. His work has received the support of a grant from the National Institutes of Health (NIH) that has been renewed yet again, with the current period extending the grant to a quarter of a century of unbroken funding.
“Since day one, no matter how crazy the ideas have been, we’ve stayed true to our central question – how do we extend the functional lifespan of the ovaries,” Tilly remarked. “This grant has allowed us to evolve that thinking over the past 20 years in ways we never would have thought about otherwise.”
This one grant has also helped Tilly secure multiple papers in top scientific journals such as Nature, Cell, Nature Genetics, and Nature Medicine, which collectively detail a spectrum of exciting advancements in the field of fertility research. Some of those advances include his discovery and purification of oocyte (egg) stem cells, and the development of new approaches to delay ovarian aging and menopause. Though his team’s work has taken some unexpected turns along the way, every project has always worked toward understanding how to sustain the functional lifespan of the ovaries in order to help females retain their fertile potential and to stay healthier longer.
Egg Preservation
The highest point of egg cell production for a human female happens well before the infant takes its first breath of air. About halfway through pregnancy, there are about 7 million immature eggs stored in the embryo’s ovaries. By the time of birth, that number has depleted to around 1 million. By the time of puberty, the ovaries contain only 200,000-300,000 eggs, having lost over 95 percent of their potential egg stockpile.
Over two decades ago, while Tilly’s lab was at Massachusetts General Hospital, he began studying the genes and pathways that controlled egg cell death to determine if his team could prevent or slow the process of egg loss. If ovary function could be extended, there would be more time in a woman’s life before menopause, which would dramatically delay the onset of the many post-menopausal health risks faced by all women as they age.
Tilly methodically assembled a molecular blueprint of the genes and pathways that help eggs survive as well as the ones that cause eggs to die, and found that egg loss occurs through a ‘natural’ cell death program termed apoptosis. During these studies, his lab also inadvertently discovered that eggs exposed to chemotherapeutic agents quickly underwent apoptosis in response to this toxin.
In light of this, Tilly readjusted his lab’s work to focus on proving that premature menopause and infertility in female cancer patients exposed to chemotherapy or radiotherapy were the result of side-effect damage to the ovaries which caused accelerated egg cell death. In 1997, his lab published their initial findings in Nature Medicine, with key follow-up studies in 2001 and 2003 in the same journal.
From there, they worked to find the key points of control that egg cells use to sense and transduce the death signal, from which they then developed a small chemical molecule that would prevent egg cells from activating apoptosis.
“Our goal was to not allow the egg to know it had been exposed to anti-cancer treatments,” Tilly said. “Through a series of studies in mice and then in monkeys, we established that if we delivered this molecule to the ovaries prior to chemotherapy or radiotherapy, the damage to the ovaries was mitigated, and ovary function and reproductive capacity were retained. If the molecule wasn’t delivered, the ovaries would fail prematurely.”
Health span vs. Lifespan
As important as this was, Tilly and his colleagues did not stop working there. Instead, they returned to their central question and dove deeper into understanding how ovaries age, and what could be done to prevent this from happening.
“Our objective was to better define key events underlying oocyte loss and ovarian aging, with a long-term goal of developing new approaches to slow the time to natural menopause, and thus delay the onset of health risks and complications tied to ovarian failure,” said Tilly.
Using a gene knockout approach, Tilly demonstrated that oocyte loss, due to aging or insults, required a protein encoded by the Bax gene. By eliminating this gene in mice, the functional lifespan of the ovaries was extended much longer, proving that by maintaining an adequate number of eggs within the ovaries, they can continue to function even as the females get very old. The results of this study were published in Nature Genetics in 1999.
Eight years later, a follow-up study published by Tilly in the Proceedings of the National Academy of Sciences USA showed the applications of this work. As women age after menopause, there are many other signs of aging due to improper ovary function. Some of these include osteoporosis, hair loss, cognitive dysfunction, cataracts, and more. Mice often show many of the same signs. But as Tilly’s Bax knockout mice grew older, they did not show these hallmarks of female aging because the ovaries continued to function much later into their lives. Equally importantly, these immense health benefits were achieved without evidence of any downsides, like increased cancer risk or incidence.
“This was getting to the central theme of something I’ve always been interested in and carried through here to Northeastern — healthy aging. It’s not to extend lifespan per say, but to extend health span,” Tilly said. “And this series of studies allowed us to show that we could safely extend health span by sustaining ovarian function, at least in mice. However, we did not know at the time how we could translate this work to humans.”
Stem cells to the rescue
As the NIH grant began to move into its second major block of funding in the early 2000s, Tilly and his team had published several papers and patents, and were continuing to explore the Bax gene and its capabilities.
To do this, they decided to directly quantify the number of eggs dying in the ovaries, which had never been done before. It had always been believed that once the egg population is endowed at birth, it starts to die off and new eggs are never made again. Therefore, any decline in the total healthy egg pool must reflect the number of eggs dying during that time period.
“As it turns out, that assumption was incorrect,” Tilly said. “We showed, quite serendipitously, that the actual rate of egg cell death was three times higher than the total net loss of healthy eggs from the ovaries.”
Suddenly, they found themselves stuck in a mathematical dilemma, because while there were many, many egg cells that were dying, that number did not match the number of healthy eggs still in the ovaries. There had to be a deposit of new eggs somewhere that hadn’t been accounted for.
“In one fell swoop, we stopped everything and rewrote the entire future of the lab based on one crazy idea,” he said. “The field and every textbook I had ever read in my life was wrong – the ovaries had the capacity to make new eggs, and we just missed it.”
In 2004 when Tilly’s lab published their groundbreaking findings in Nature challenging the belief that females, once born, cannot make new eggs, they took a lot of negative criticisms about their discovery since it looked to overturn what most scientists had believed to be true for more than fifty years.
But the NIH stayed true to Tilly’s pursuit of the basic question about ovarian aging, and continued to fund Tilly’s research on the idea that a rare population of previously unknown stem cells must be responsible for making new eggs. In 2012, his lab succeeded in purifying oocyte stem cells from the ovaries of reproductive age women, showing in a Nature Medicine article that even as adults, women’s ovaries have the capacity to make new eggs. This breakthrough was followed this year by a publication in Nature’s Scientific Reports from Tilly’s lab firmly establishing that oocyte stem cells not only exist but also contribute directly during adult life to the pool of eggs used by females to reproduce.
Through studying these oocyte stem cells, Tilly and Assistant Professor of Biology Dori Woods, along with their team of undergraduate and graduate student trainees in The Laboratory for Aging and Infertility Research (LAIR) at Northeastern, are now collaborating to ‘build’ a fully-functioning artificial human ovary outside of the body that is composed of cells completely matched to a given person’s genetic identity.
“In effect, we would have a way to produce an essentially unlimited number of healthy new eggs from this patient-matched artificial ovary, thereby removing the need for one of the most expensive and painful aspects of in-vitro fertilization (IVF) – months of hormone injections which produce only a few eggs for the doctor to attempt IVF with,” said Tilly. Thinking even more broadly, Tilly also views this artificial human ovary as a potential means to obtain the same health-promoting outcomes in women approaching menopause as those achieved in the aging Bax knockout mice. “The benefits of sustained ovarian function,” he explained, “whether it be through knockout of a gene that drives egg cell death in mice or through reintroduction of artificial ovarian tissue initially engineered outside the body in women, should produce the same beneficial outcomes for the aging female body.”
New energy for tired eggs
In addition to building an artificial ovary, Tilly has also spent his time working to fix one of the grand challenges in human female fertility — the deterioration of egg and embryo quality with age that leaves many women unable to conceive, even through IVF. Tilly’s lab built a technology called AUGMENT (autologous germline mitochondrial energy transfer), which uses a woman’s oocyte stem cell mitochondria as fully-charged “batteries” to reinvigorate her tired eggs. After isolating a small amount of ovary tissue, collecting the oocyte stem cells and extracting their mitochondria, these batteries are a perfect genetic match that can be injected back into the same woman’s eggs at the time of IVF. Tilly and Woods patented the technology, which was licensed to OvaScience, a start-up company Tilly co-founded in 2011. They have since moved his technology into clinical studies at several leading IVF centers around the globe.
In 2015, Zain was the first baby born through AUGMENT to thrilled parents at the Toronto Center for Assisted Reproductive Technologies, thanks to Tilly’s research. Another woman in Dubai had attempted to become pregnant but could not, enduring 16 failed IVF cycles. Through AUGMENT, she finally achieved her dream of having a child of her own.
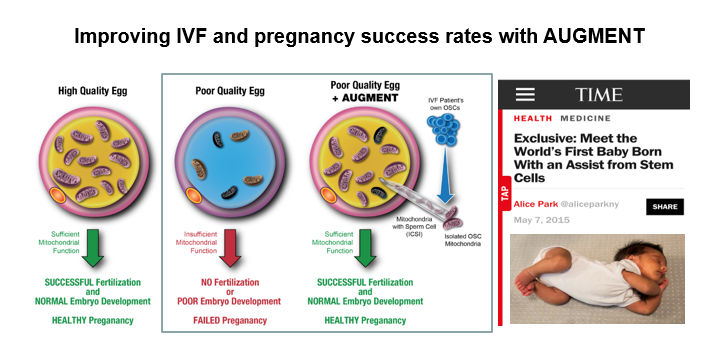
Diagram courtesy of Jonathan Tilly.
“Outcomes are what matter to us,” said Tilly. “People can debate whether oocyte stem cells exist all they want, but what we really care about is delivering safe and effective new technologies for women in need. If the stem cells didn’t exist, this stuff wouldn’t be happening.”
It’s been a long road for Tilly since his initial dogma-breaking paper in Nature in 2004, but clearly his dedication and perseverance have been worth it, and more is on the horizon.
Next steps
As the grant now continues to fund the next five years of his work through 2022, Tilly and members of the LAIR are taking their research in a new direction, but still staying true to the overall goal of sustaining the functional lifespan of the ovaries.
The group looked at women’s ovaries throughout every decade of life, including post-menopause, and saw that even after menopause, the ovaries still have oocyte stem cells — they’re just dormant. The lab published some of these findings in 2015 in Seminars in Reproductive Medicine. If the stem cells are removed from the body, they’re still capable of egg production, so there must be something happening in the ovaries that causes this dysfunction.
So now, Tilly and his team are exploring the field of mechanostimulation, looking at the effect of mechanical forces on the ovaries and egg production. Their theory is that as women age, it is more difficult for their ovaries to transduce mechanical signals to the oocyte stem cells. The older the ovaries are, the tougher and less flexible they are. To test this idea, Tilly’s lab is working with a specialized machine that allows tissues or cells to be ‘stretched’, with certain amounts of pull in different directions. By determining how much the ovaries might be stretched during their normal function in the body, as they experience constant changes in force through follicle growth and ovulation, they can apply that same amount of stretch to oocyte stem cells in a dish to see if this triggers the production of new eggs.
“Our hypothesis is that if the ovaries become less able to stretch, the stem cells aren’t getting that stimulus anymore so they’re not told to make new egg cells,” Tilly said. “We think one of the key things going on in ovaries is that the microenvironment the oocyte stem cells reside in becomes compromised or unsupportive to the cells as women age.”
If his hypothesis is proven correct, Tilly believes that there is great opportunity for development of a new device that would enable the ‘stretch signal’ to be reintroduced into aged ovaries as a means to restore egg production. While it’s still in the very early stages, Tilly is still very optimistic that this new line of work will yield big outcomes for science and for women.
Two decades later
Tilly cannot thank the NIH’s National Institute on Aging enough for the continuity of research that this grant has provided him and his colleagues since it was first activated in 1995.
“We’ve found many ways over the years to look at that one central question,” Tilly said. “How does aging of the ovaries happen and how can we intervene, either naturally or through some other means?”
Their work has answered that question in many directions and applications, and they continue to delve even further. And for Tilly, he’s happy to take on that challenge every day he comes into work.
“I get to do what I love, I get to discover,” Tilly remarked. “I get to actually achieve what I’ve always wanted to do, which is to make my work meaningful. The smile on the faces of baby Zain’s parents, holding him in their arms, was just off the charts. That’s why I’m committed to the work that we do. It is designed to change people’s lives for the better, and for some that has already happened.”

