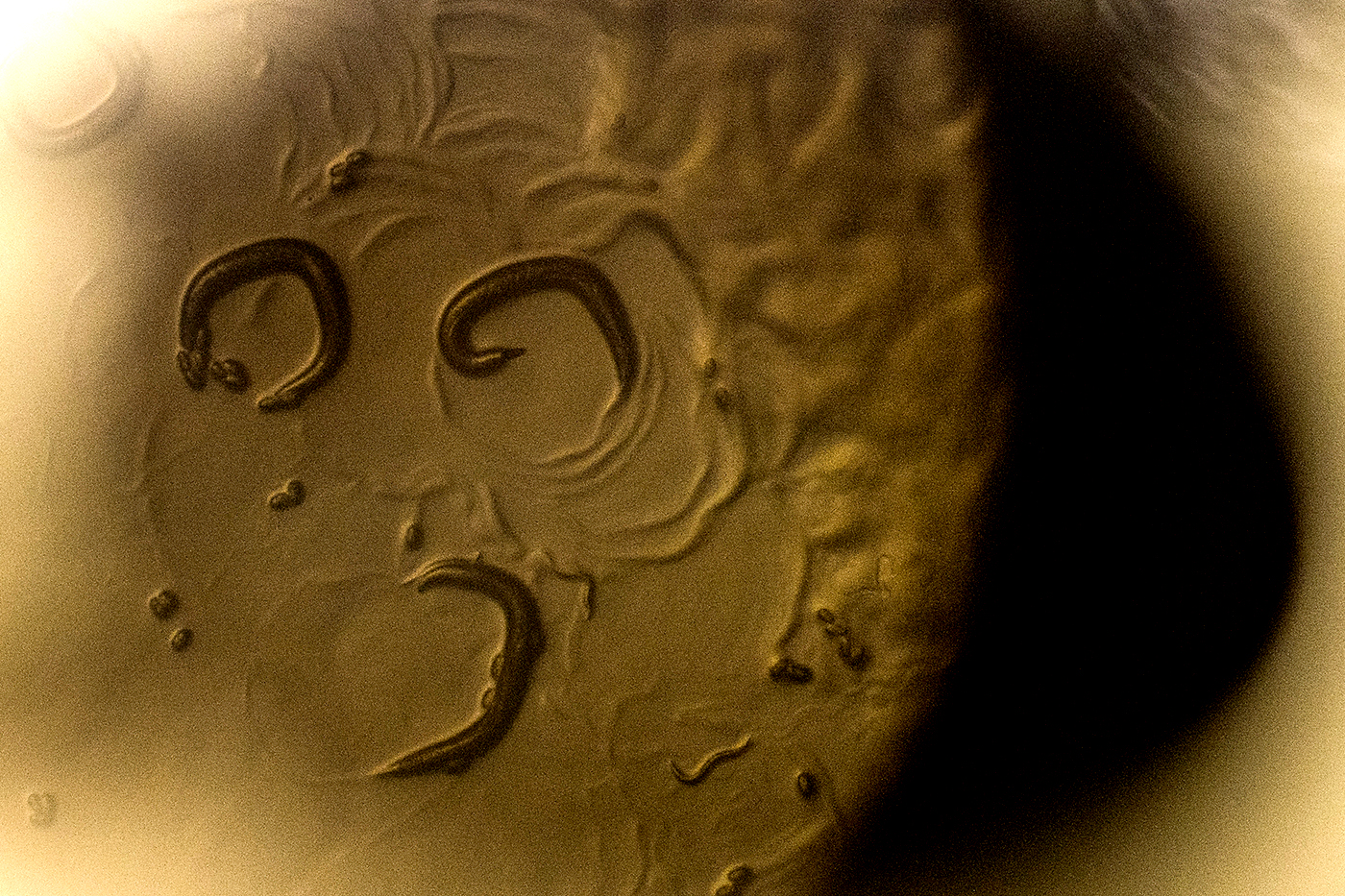
Looking for a simple organism as a subject to learn more about the basic functionality of multi-cellular organisms, the biology community adopted the nematode C. elegans. This microscopic worm has become, arguably, one of the most comprehensively understood organisms. Yet, little is known about how they deal with conflicting environmental cues and how that shaped their evolutionary track. A new study from a Northeastern-led collaboration looking into the worms’ interactions with their microbiome found that the worms are willing to leave a bacterial food source when they’re exposed to one of their deadliest toxins, hydrogen peroxide. The team then identified the driving neurological mechanism behind the worms’ choice to jump ship.
“It started as a bet in the lab,” Northeastern biology professor Javier Apfeld says. Jodie Schiffer, a graduate student at Apfeld’s lab, noticed that C. elegans’ would turn off their defenses against peroxide when in the presence of bacterial food; however, only some species of bacteria are capable of depleting peroxide in the environment and consequently protecting the worms. So, the question was: were the worms guessing that the bacteria they found were the protective type, or did they know? Stephanie Stumbur, a health science undergraduate, began developing experiments to put the bet to the test in what would culminate in the graduate-undergraduate duo co-authoring the paper.
The team ran a series of experiments where they presented groups of worms with choices between a combination of environments with and without peroxide present and bacteria that protected the worms from peroxide and bacteria that did not. Schiffer and Stumbur identified that the C. elegans immediately recognized and had a negative response toward peroxide and would frequently leave a food source upon its detection.
“They’re pretty preliminary animals, and food is a necessity of life,” Stumbur says. “The fact that they were making the choice to leave the food source, that was a pretty significant thing that not a lot of people would have expected.”
However, not all the worms would leave the toxic environment when food was present, indicating there must be some evolutionary validity to a worms’ choice to stay. This meant that, in some instances, the likelihood of the environmental conditions improving could be greater than the likelihood of finding a new food source, should the worm leave.
“They are hedging,” Apfeld says. “Yes, the environment now is terrible, but maybe a snail will come by and poop some bacteria that degrades peroxide and remakes the environment for the worm.”
To understand the underlying mechanisms in the brain to explain the behavior, the team set out to image the worms’ neuron activity when prompted with bacteria and peroxide stimuli. Northeastern physics professor Vivek Venkatachalam and his group, a frequent collaborator with the Apfeld lab, helped with the imaging process.
They found that not only did some neurons respond strongly to food, as expected, but other neurons also responded to hydrogen peroxide. Additionally, both responses muted one another: Recognition of peroxide muted the worms’ bacteria response, and bacteria that degraded hydrogen peroxide prevented the worms from sensing any peroxide.
Importantly, the work shows hydrogen peroxide, a toxin for many forms of life, can alter the function of a sensory system. This means, in biological systems, peroxide can both act as a damaging agent at one level, and a sensory messenger or input at another. Working with the well-known C. elegans allowed the group to take advantage of a controlled environment to learn about fundamental sensory mechanisms that sustain life. This, in turn, provides insights into attempts to understand the mechanisms of more complex organisms in less-controlled environments.
“We can learn the basic rules of how complex living systems work and how their strategies evolved,” Apfeld says. “Some of the strategies that the worms use may be worm specific … some of them may be general.”
During the late stages of the project, the Apfeld lab reached out to groups at Yale and Baylor universities for help characterizing different peroxide-protective and -nonprotective bacteria used in the study. The groups immediately offered support in the form of the analyses of over 180 bacteria species.
“It’s so nice to work in this community, the C. elegans community,” Apfeld says. “They’re so generous that people just volunteer to do us a favor.”
Stumbur started in the lab her first year as an undergraduate and, through the mentorship of Schiffer and Apfeld, progressed to larger projects and contributions while working part-time during semesters and full-time during summers and a co-op at the lab. To cap the experience, Stumbur presented the research at the 2021 international C. elegans conference. Stumbur, who is pursuing medical school for a career in healthcare and public health, feels her experience at the lab helped prepare her for her future in health. She completed a capstone project for her degree that consisted of a literature review looking at how the microbiome affects the human body.
“I was really able to get another perspective,” Stumbur says. “I want to go into medicine, which is a little bit different than the research I was doing, but through my capstone, I was able to find the harmony of how does health — how does medicine — connect to the research I was doing?”
Stumbur and Schiffer both graduated with their respective degrees in the spring of 2021, but not without leaving a mark. Their work shifted the trajectory of the Apfeld lab toward the new and promising field of microbiome research. Of the research culture that made this project possible — with collaboration between undergraduate and graduate students and other labs both inside and outside of Northeastern — Apfeld says, “It’s part of how we do things at Northeastern.