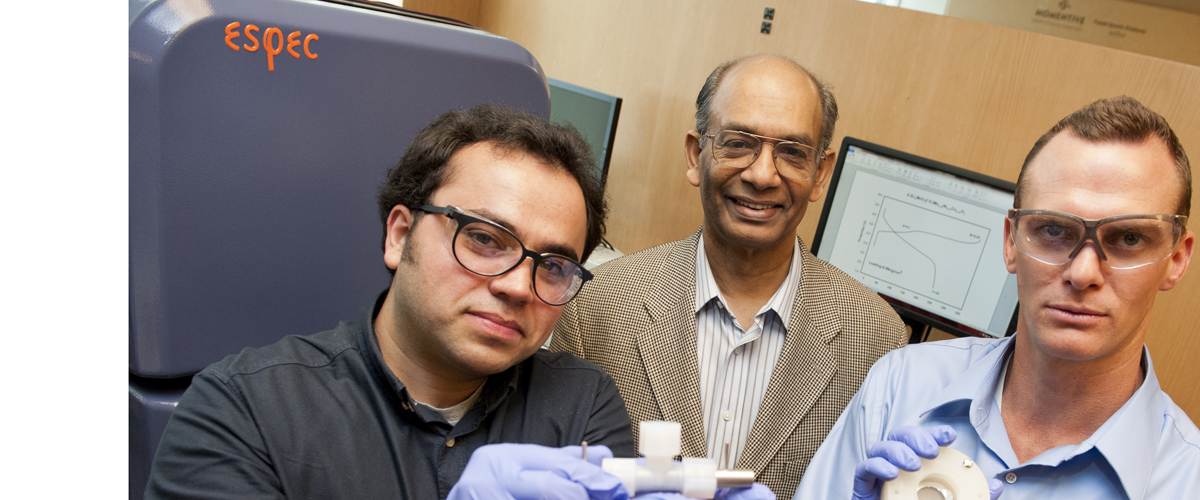
by Angela Herring
When he was a kid, Mehmet Ates would take the dead batteries from his family’s appliances and crack them open with rocks in the back yard. “I wanted to figure out how they worked,” he said.
The standard nickel-metal hydride batteries, however left the future chemist disappointed: once opened, they revealed nothing but a fine black powder.
Matthew Trahan never split open batteries, but he did have a strong appreciation for the outdoors and his environment. As a chemistry student in Missouri he realized he wanted to change society by improving the technologies essential to the development of electric vehicles.
Today, Ates and Trahan, both graduate students in the same lab, are working with research professor K. M. Abraham to develop the next generation of energy efficient lithium batteries. One of Ates’ childhood batteries would have to be five to 20 times larger in order to store the same amount of energy as one of the teams’ experimental batteries, he explained.
Recognized with the physical and life sciences award at the Research, Innovation, Scholarship, and Entrepreneurship expo earlier this year, the duo is working in two arms of Abraham’s lab. Ates is developing a next-generation lithium-ion battery—a lithium rich version of the batteries that today power everything from cell phones to electric vehicles. As the lightest element available for battery production, lithium provides an extremely efficient venue for energy storage. “One gram of lithium contains many more atoms”—the energy storing unit in the material—“than one gram of nickel,” explained Ates.
Commercial lithium-ion batteries currently are composed of a lithiated graphite anode and a cobalt oxide cathode. Next generation, “lithium rich” batteries, which research groups around the world are studying, use manganese-oxide instead. Abraham’s team is adding a metal compound to the system to increase the energy retention capacity of this already-better battery by 40 percent.
But still, even the best-case scenario for the lithium-rich batteries isn’t satisfactory for Abraham. “My brother lives in Philadelphia, 304 miles away,” he said. “That takes five or six hours to drive.” If he wants to drive an electric car there today, he’d have to stop at least once during the trip to charge the vehicle—a process that can take many hours, he said.
In 1996, Abraham published a paper demonstrating a new kind of lithium battery that uses plain old air as the cathode, instead of cobalt– or manganese-oxide. Nearly two decades, this is the battery Trahan is working on now.
With increased government pressure to develop clean energy alternatives, people are finally realizing the utility of the so-called lithium-air battery. There is a worldwide effort to perfect this battery, capable of storing five to 10 times more energy than the lithium rich manganese-oxide batteries, and 20 times more energy than the standard nickel-metal hydride battery.
“We are revolutionizing lithium-ion and lithium air technology,” Abraham said.